UX/UI DEsign | Healthcare Digital Experience | Horizon Therapeutics
CLINICAL TRIALS RESOURCE HUB
A collection of UX projects for Horizon Therapeutics, each designed to bring warmth, clarity, and trust to complex healthcare systems through intentional, human-centered design.
my ROle
UX Research | UX Strategy | Information Architecture | Wireframing & Prototyping | Cross-Functional Collaboration
Tools
XD, Crownpeak
Timeline
12 weeks
Deliverables
Competitive and heuristic analysis, phased content and IA strategy, high-fidelity wireframes and responsive page design, component documentation, and CMS implementation guidance (Crownpeak)
OVERVIEW
As part of Horizon Therapeutics’ enterprise UX modernization effort, I led the creation and design of the company’s first dedicated Clinical Trials Hub — a scalable framework to help patients, caregivers, and healthcare professionals discover current and upcoming research opportunities directly through Horizon’s ecosystem.
01
Previously, users were directed off-site via external links to third-party trial databases, resulting in fragmented navigation, inconsistent branding, and reduced engagement.
This initiative established a cohesive, in-house experience for the first time.
CHALLENGE
Before this project, no centralized page existed to communicate Horizon’s clinical research or guide users to relevant trials.
The experience relied solely on outbound links to third-party databases — creating gaps in context, trust, and accessibility.
Internally, there was a need to:
Convey complex medical information in clear, user-friendly language
Comply with strict regulatory guidelines
Build a flexible, modular framework that could evolve with new data
02
GOAL
The focus was to balance clarity and credibility, ensuring scientific accuracy while creating a calm, trustworthy interface for patients and caregivers.
To design a unified Clinical Trials experience that:
Informed users about Horizon’s ongoing studies and approach to research
Simplified access to external databases while maintaining brand continuity
Established a scalable, component-based foundation for future expansion
03
PROCESS
Collaborating closely with medical communications, clinical operations, and development teams, I:
Audited competitor and biopharma benchmarks to identify best practices
Defined content hierarchy and patient-centered language patterns
Prototyped phased layouts in XD to test clarity and readability
Partnered with Crownpeak developers to identify feasible components and ensure compliance
04
RESEARCH FINDINGS
User feedback revealed key insights that guided the phased rollout:
Users sought a single, authoritative source of information about Horizon’s studies
External links created context gaps and confusion about Horizon’s role in the trials
Internal teams needed a flexible CMS structure to scale future content
05
STRATEGIC SOLUTION (Phased Rollout)
To align with evolving content and technical constraints, I proposed a four-phase implementation:
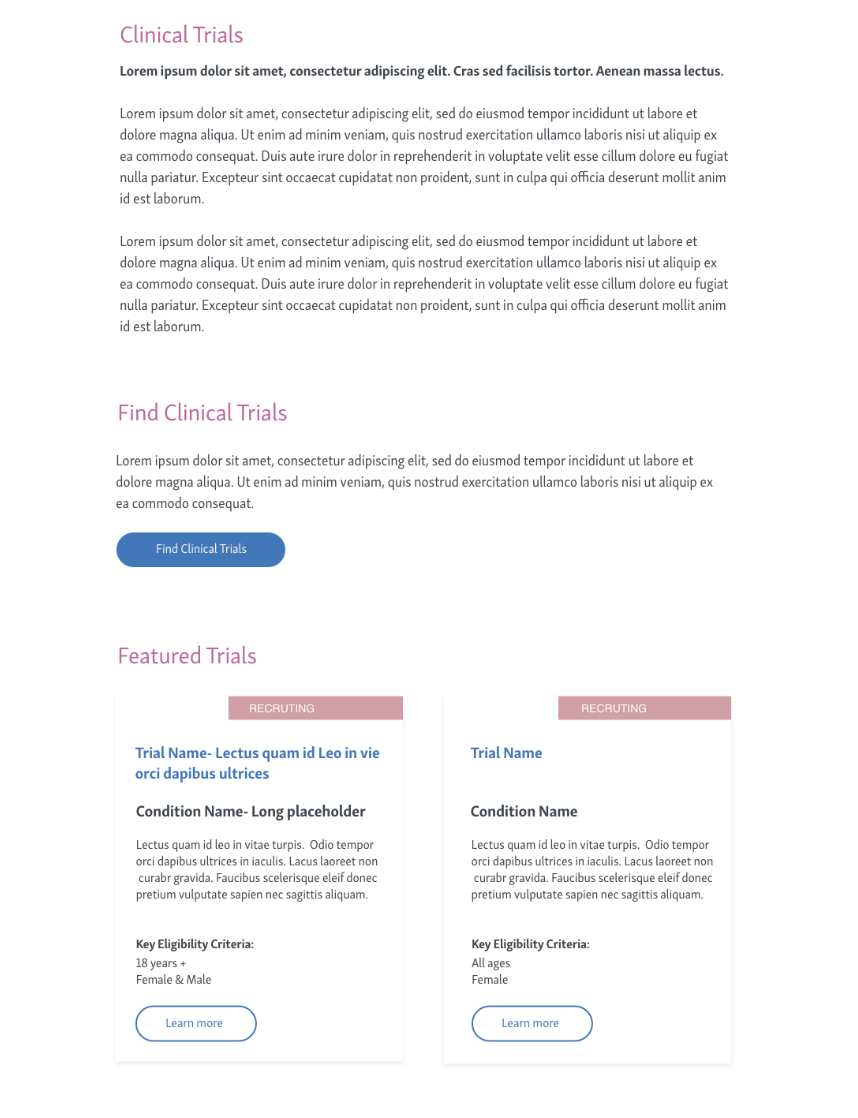
Phase 1: Launch a single-page overview highlighting 2–3 featured studies and FAQs.
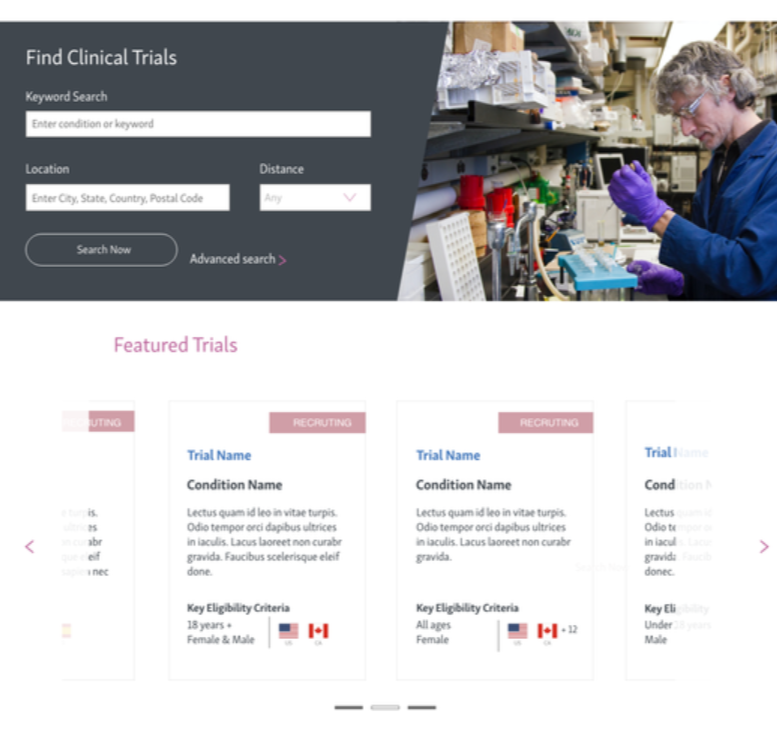
Phase 2: Introduce a card carousel for featured studies with detail pages.
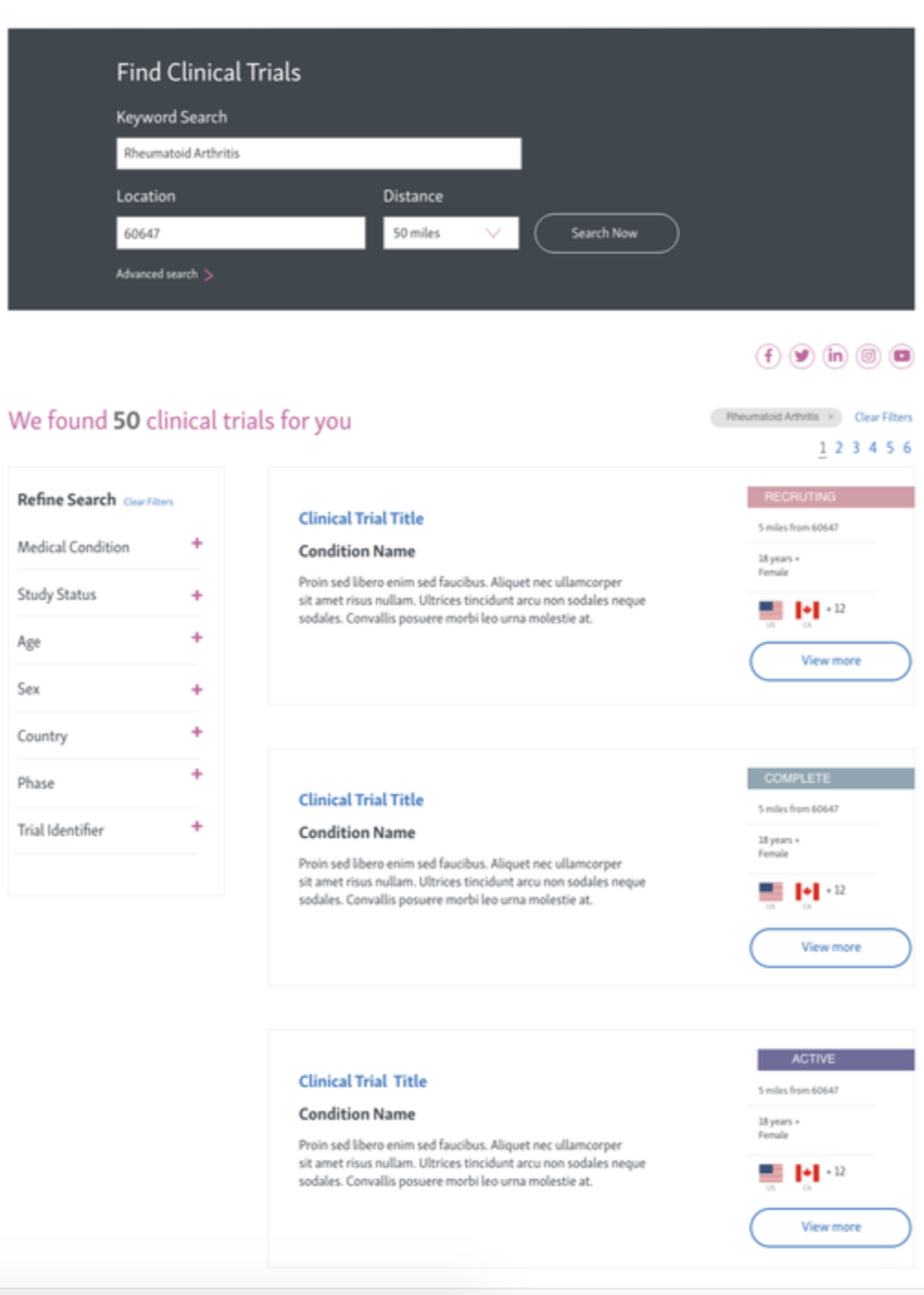
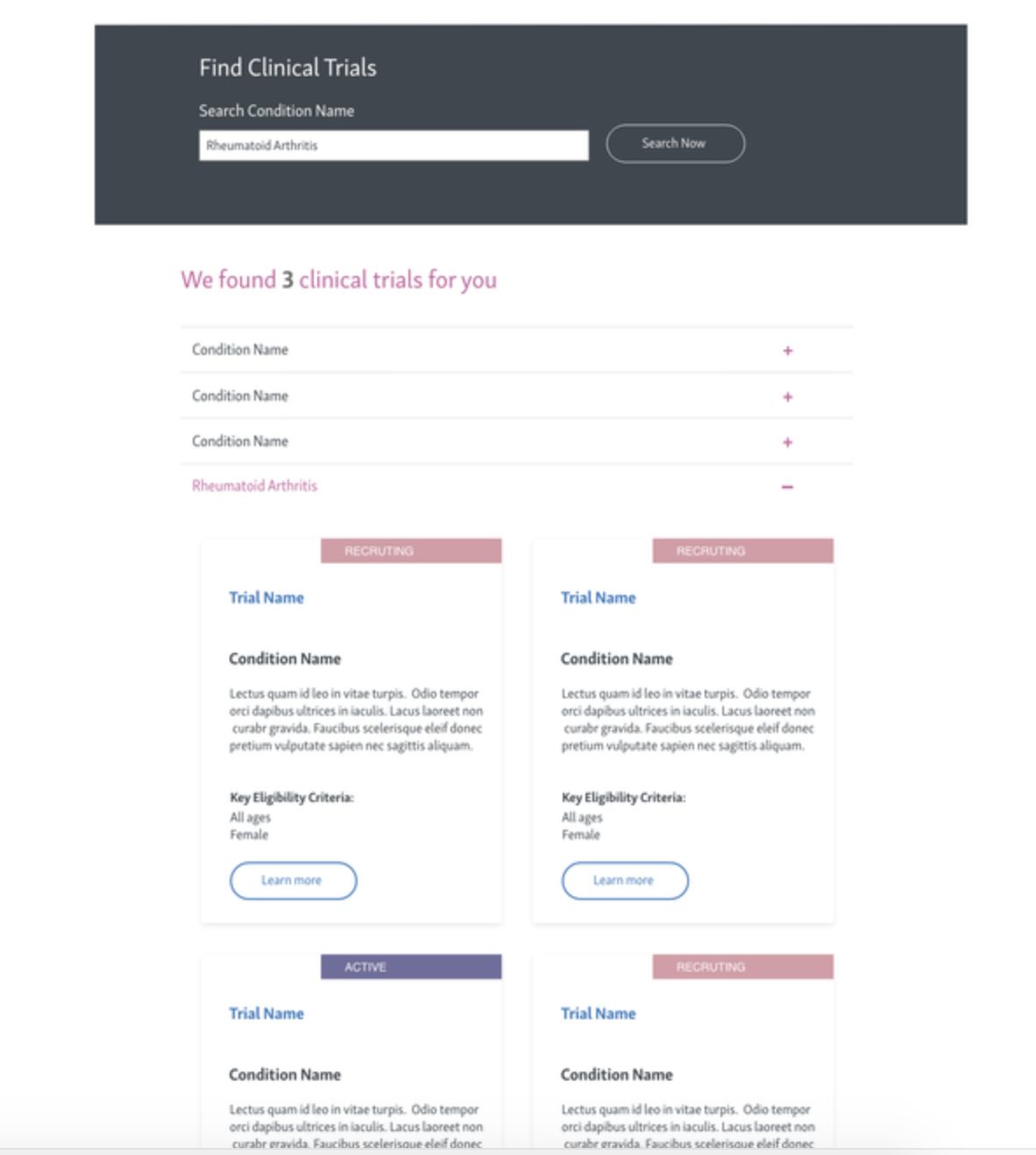
Phase 3: Integrate a search component for easier discovery.
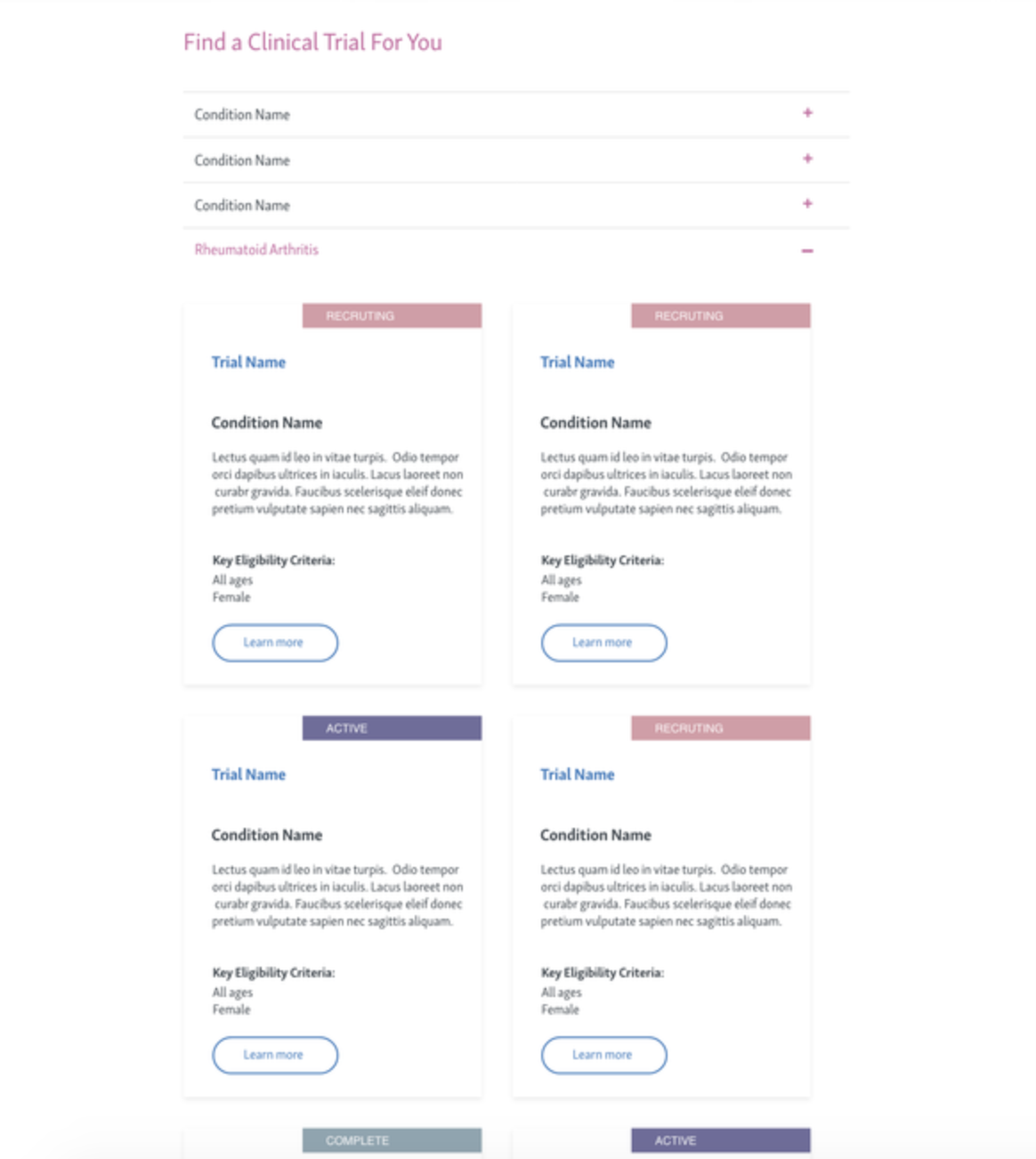
Phase 4: Add filtering tools for refined navigation by therapeutic area and eligibility.
This approach allowed continuous testing and iteration without disrupting live release timelines.
06
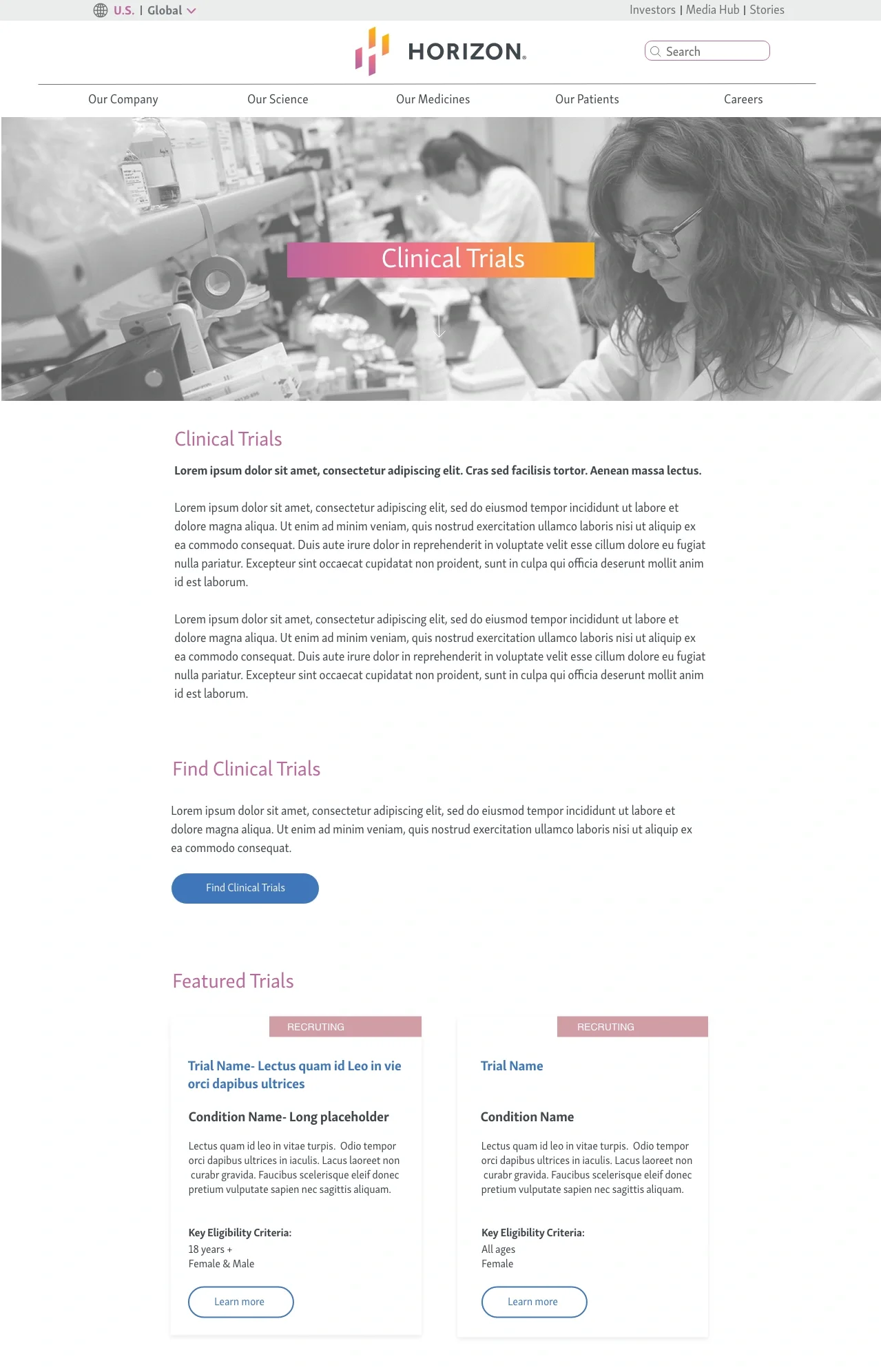
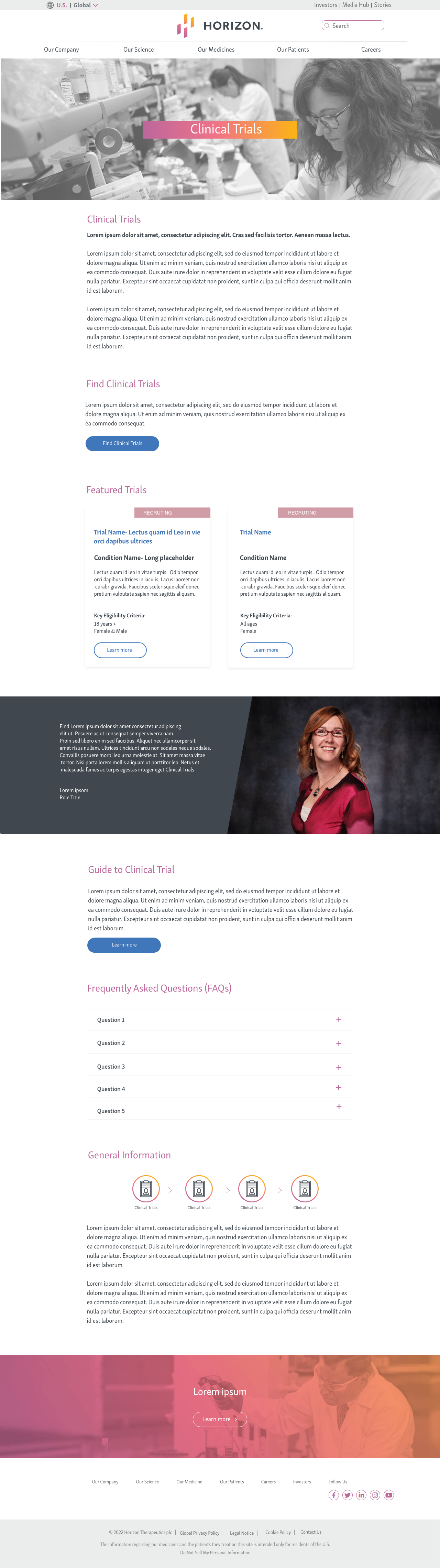
phase 1 - HOmepage
HOmepage
phase 2
phase 3
phase 4
TRials list page
phase 2
phase 3
phase 4
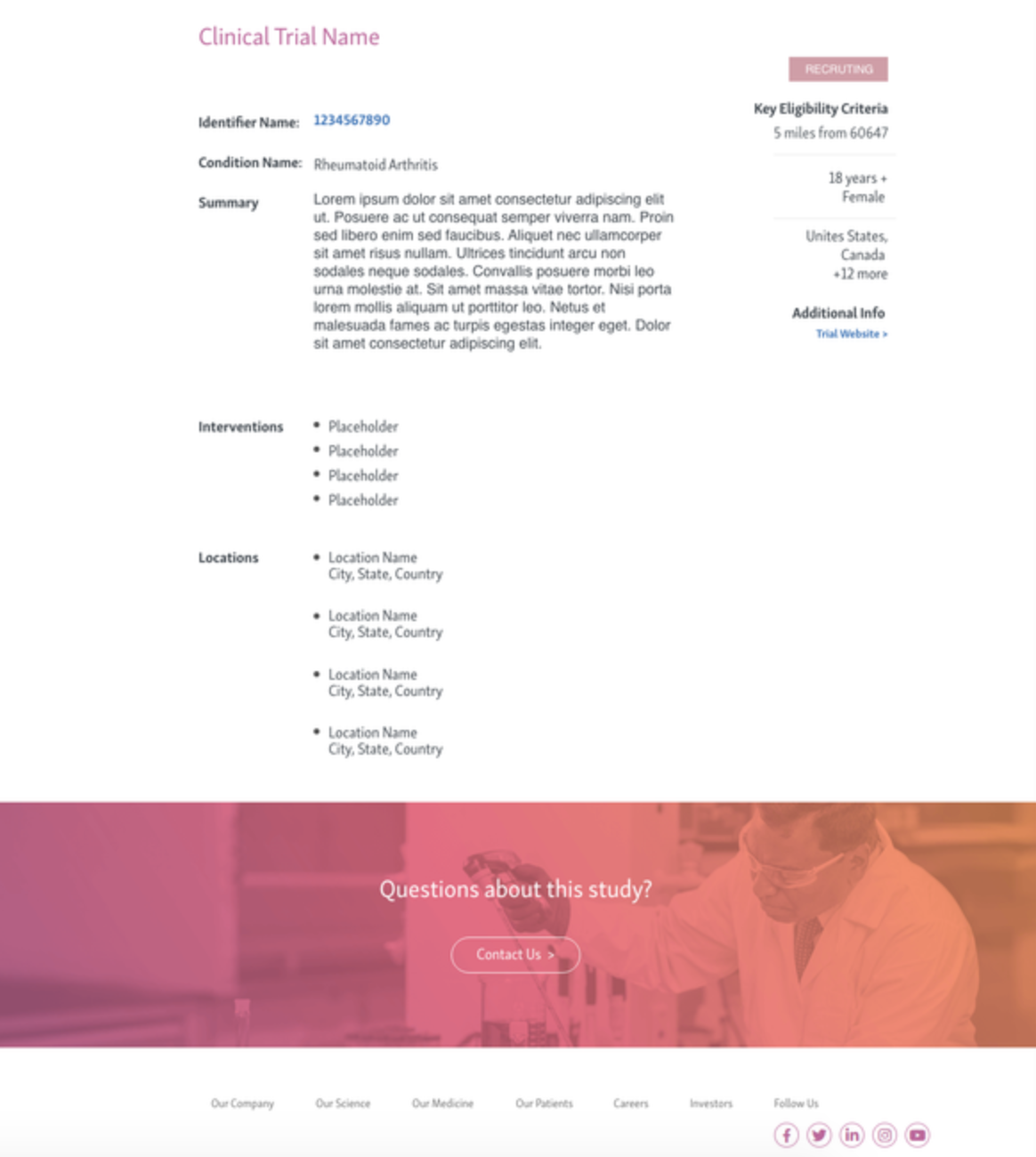
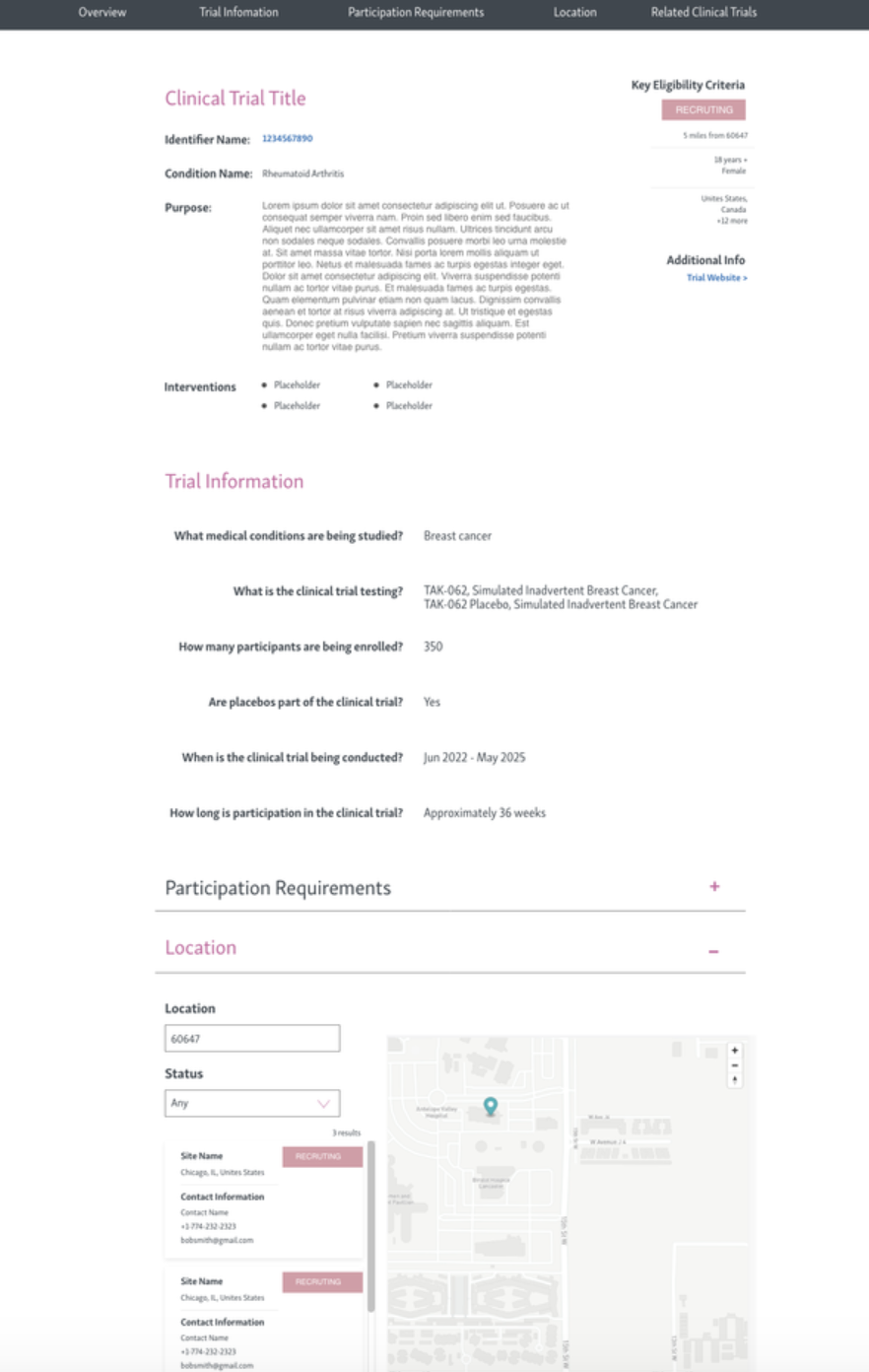
TRials details page
phase 2
phase 3
phase 4
IMPLEMENTATION
I designed a scalable UI system with consistent spacing, iconography, and typographic hierarchy — all aligned to Horizon’s design standards.
Each phase was built to layer seamlessly, enabling future enhancements (search, filters, dynamic cards) without structural redesign.
Worked closely with developers to define reusable modules within Crownpeak, ensuring accessibility and regulatory compliance.
07
Top of Homepage
Bottom of Homepage
OUTCOME
The new Clinical Trials hub:
Created Horizon’s first on-site framework for clinical research communication
Improved discoverability and engagement by maintaining users within Horizon’s experience
Set the standard for content hierarchy, layout, and interaction design used in later site initiatives
It became a model for how Horizon could internalize third-party experiences while preserving credibility and brand trust. Also, it improved discoverability and user retention on the site by consolidating clinical resources in one place.
08
REFLECTION
This project demonstrated how user-centered design and phased implementation can bridge gaps between content strategy, regulatory compliance, and technical systems — creating scalable impact under real-world constraints.
It also showcased the power of UX leadership to create clarity and connection where no prior framework existed.
09
FUTURE STATE
Following the acquisition of Horizon by Amgen, the framework and design logic were retained and documented as part of Amgen’s broader digital ecosystem—ready for adaptation within its unified enterprise experience.
Work completed under Horizon Therapeutics Digital Experience team prior to Amgen integration.